Activation Energy Is Best Described as
If the activation energy is low rate will be high. KJ and the reaction is.
What Is Difference Between Endothermic And Exothermic Reaction If Both Require Activation Energy Quora
KJ and the reaction is endothermic.

. If there is no activation energy no reaction will occur. 1energy input needed to break bonds of reactants. The energy level of the reactants.
Which of the following best describes the activation energy of a reaction. 3White phosphorus has a lower activation energy than red phosphorus. Activation energy is described as the minimum kinetic energy needed to start a reaction yet it is found on a potential energy graph.
As well it mathematically expresses the relationships we established earlier. But when we add a catalyst into the reaction then there will be a decrease in activation energy and as a result molecules will lesser amount of energy are able to participate in the reaction. The energy threshold that must be reached before a reaction can proceed and products may be formed.
This is the starting energy before we go on the reaction. 4Products have higher chemical potential energy than reactants. Energy of activation The energy of activation is best described as Multiple Choice the speed at which a reaction proceeds to form products.
Which Statement Best Describes The Relationship Between Activation Energy And Rate Of Reaction. Activation energy can best be described as. The difference in energy between reactants and the maximum energy.
Activation energy is needed so reactants can move together overcome forces of repulsion. BYJUS Online learning Programs For K3 K10 K12 NEET. Activation energy is defined as the minimum amount of energy required by the reactant molecules to undergo a chemical reaction.
The answer is B as activation energy is the least possible amount of energy minimum which is required to start a reaction or the amount of energy available in a. Activation energy is the minimum amount of energy needed to start a chemical reaction. So which one is it.
KJ and the reaction is C. Why is activation energy important. The activation energy is 50.
A protein that speeds up a chemical reaction. The diagram in figure 13 shows the amount of energy the reactions starts and how much energy is being released or absorbed depending on the reaction that is occurring. The maximum energy level of the reaction.
Ad by The Penny Hoarder. See the answer See the answer done loading. Group of answer choices a The amount of energy lost in an exothermic reaction.
The activation energy in the Arrhenius equation can best be described as asked Jun 26 2017 in Chemistry by maju88 a. There is no relationship between activation energy and rate of a reaction. The best statement that describes the energy of activation is that it is more quickly overcome when the temperature increases.
And if the energy of the colliding molecules is less than the activation energy no reaction will also occur. What is the relationship between activation energy and rate constant. Activation energy is increased by as much as 4 per second leading to more rapid reactions.
Reducing the activation energy can increase the rate of a reaction. Which of the following best describes the activation energy of a chemical reaction. Activation energy can best be described as.
Activation energy is best described as ___ the energy required to initiate a chemical reaction Rank the grades of coal by their relative desirabilities starting with mos desirable at the top. How do you define rce energy and rate of reaction. T he Arrhenius equation allows us to calculate activation energies if the rate constant is known or vice versa.
Activation energy is the energy barrier that has to be crossed by reactants to reach transition state in order to form products. The minimum amount of additional energy needed by a reacting molecule to get transformed into the product is termed activation energy. The difference in energy between reactants and the maximum energy.
KJ and the reaction is D. B The amount of energy gained in an endothermic reaction c All of the above d The amount of energy required to start a reaction. Because activation energy is the amount of energy required for a chemical reaction to take place.
None of the answer choices are correct. So I think its C. The difference in energy between reactants and products.
The best definition of activation energy is that activation energy is the energy required to bind a substrate to an active site option C. The difference in energy between reactants and products. The activation energy is 10.
The Activation Energy can best be described as. Decreasing the activation energy. 2energy stored in chemical bonds.
Activation energy can be described as the a energy of motion b energy of the from CHEM 12 CHEM 12 at University of British Columbia. The activation energy is 10. The activation energy is 50.
Science Chemistry QA Library Which statement below best describes the relationship between activation energy and the rate constant. The energy level of the products. 1 on a question.
When 10 mL of A is added to 10 mL of B the reaction takes twenty seconds. Which statement best describes the relationship between activation energy and rate of reaction. Activation energy is the energy absorbed before it can start a chemical reaction.
A numerical description of the amount of energy needed by colliding reactant molecules in order to form. Up to 24 cash back Which statement correctly describes the energy changes that occur in the forward reaction. As activation energy term Ea increases the rate constant k decreases and therefore the rate of reaction.
A rapid reaction happens very rapidly as it has very low activation energy. O As the activation energy increases the rate constant decreases O As the activation energy increases the rate constant decreases O The activation energy and rate constant are both dependent upon temperature O As the activation energy. All chemical reactions including exothermic reactions need activation energy to get started.
Increasing the activation energy can increase the rate of a reaction.

Activation Energy Definition Formula Si Units Examples Calculation
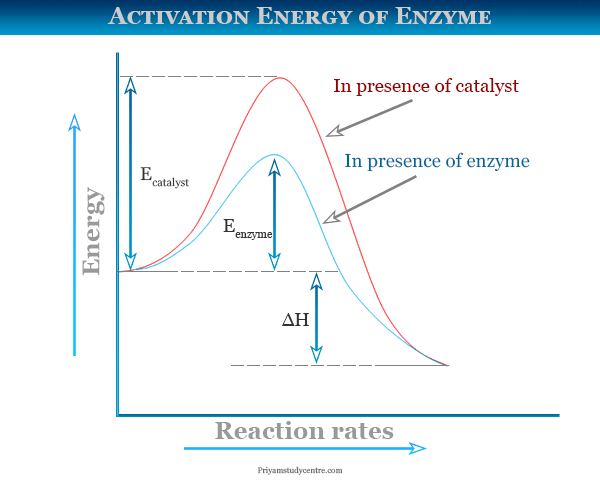
Activation Energy Definition Formula Diagram Examples

Activation Energy Article Khan Academy

Activation Energy Definition Formula Si Units Examples Calculation

Activation Energy Definition Formula Si Units Examples Calculation

Kinetics Why Is Activation Energy Drawn In A Potential Energy Diagram In Reactions Chemistry Stack Exchange

Activation Energy Definition Formula Si Units Examples Calculation

Activation Energy And Temperature Dependence Boundless Chemistry

Is Activation Energy Negative Or Positive
Does Activation Energy Change With Temperature Quora
Activation Energy And Temperature Dependence Boundless Chemistry

Potential Energy Diagrams Ck 12 Foundation

1 The Graph Below Represents The Potential Energy

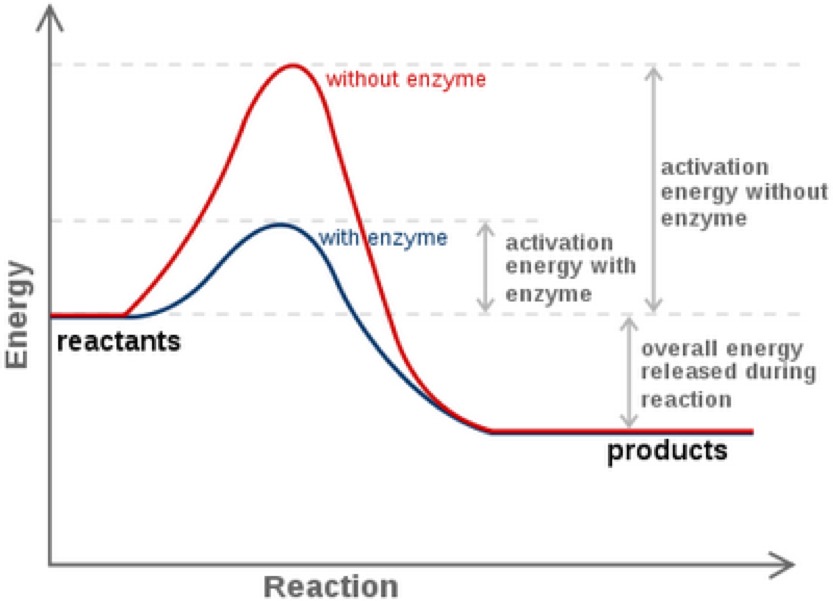


Comments
Post a Comment